NOW APPROVED COTELLIC in combination with ZELBORAF® (vemurafenib)

- Amanda Bridges
- May 4, 2016
- Drugs, Patient Assistance

Genentech BioOncology® Access Solutions and Patient Partners™
Genentech BioOncology Access Solutions provides reliable, effective access and reimbursement services to assist your patients and practice. We can help address the needs of each patient’s coverage scenario.
Available assistance for your eligible patients*:
- Patient situation
- Patient assistance option
- Insurance status of potentially eligible patients
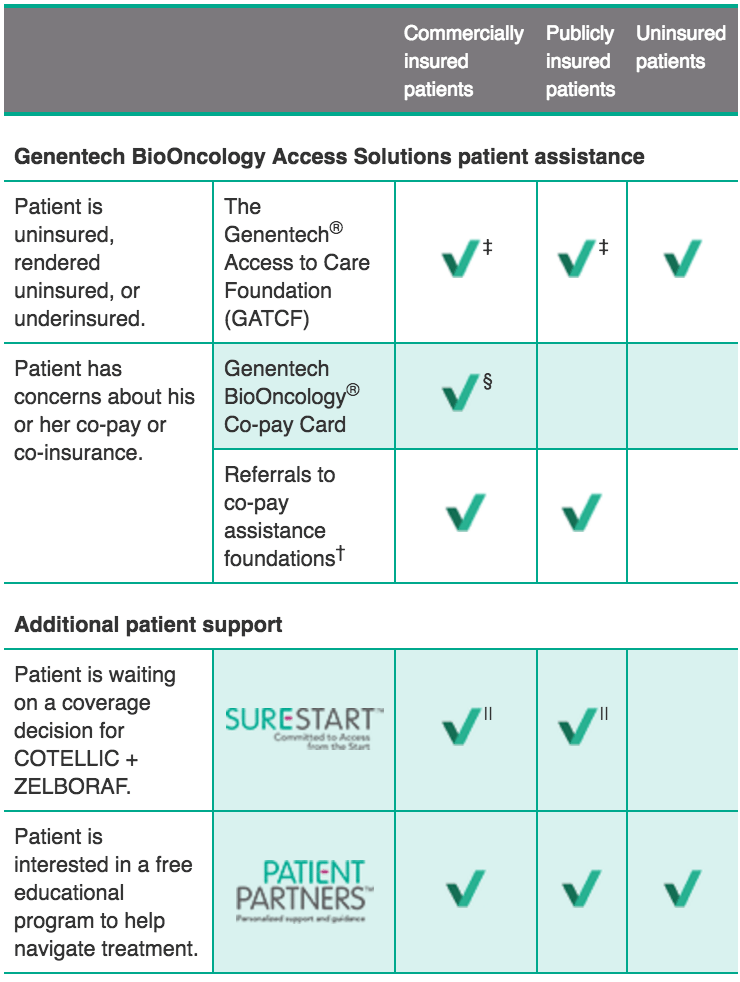
† Genentech does not influence or control the operations of these co-pay assistance foundations, but Genentech BioOncology Access Solutions can assist patients in navigating the process of seeking co-pay assistance by making an appropriate referral based on a patient’s diagnosis and by assisting with the application process. We cannot guarantee co-pay assistance once a patient has been referred by Genentech BioOncology Access Solutions. The foundations to which we refer patients each have their own criteria for patient eligibility, including financial eligibility.
For more information on patient assistance for COTELLIC + ZELBORAF, call Genentech® Access Solutions at (888) 249‑4918 or visit Genentech-Access.com/BioOncology.


* Programs have specific eligibility criteria.
‡ To be eligible for free medicine from GATCF, insured patients must have exhausted all other forms of patient assistance (including Genentech brand-specific co-pay cards and support from co-pay assistance foundations supporting the patient’s disease state) and meet additional financial and medical criteria.
§ To be eligible for the Genentech BioOncology Co-pay Card, patients must have commercial insurance and meet other criteria.
|| To be eligible for Sure Start, patients must have insurance and their plan must require a prior authorization. Other terms and conditions apply. Free shipments can continue for up to 3 months for clinically appropriate patients if a coverage decision is still pending. Visit COTELLIC.com for more information.
INDICATIONS AND USAGE
COTELLIC (cobimetinib) is indicated for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in combination with ZELBORAF (vemurafenib).
Limitation of Use: COTELLIC is not indicated for treatment of patients with wild-type BRAF melanoma.
IMPORTANT SAFETY INFORMATION
Review the Full Prescribing Information for ZELBORAF for information on the serious risks of ZELBORAF.
WARNINGS AND PRECAUTIONS
The following can occur in patients treated with COTELLIC
- New primary malignancies, including cutaneous and non-cutaneous malignancies
- Hemorrhage, including major hemorrhages
- Cardiomyopathy, defined as symptomatic and asymptomatic decline in left ventricular ejection fraction
- Severe dermatologic reactions, including rash and other skin reactions
- Serous retinopathy and retinal vein occlusion
- Hepatotoxicity
- Rhabdomyolysis
- Severe photosensitivity
- Embryo-fetal toxicity
Most Common Adverse Reactions
- The most common (≥20%) adverse reactions with COTELLIC were diarrhea, photosensitivity reaction, nausea, pyrexia, and vomiting. The most common (≥5%) grade 3-4 laboratory abnormalities are increased GGT, increased CPK, hypophosphatemia, increased ALT, lymphopenia, increased AST, increased alkaline phosphatase, and hyponatremia.
You may report side effects to the FDA at (800) FDA‑1088 or www.fda.gov/medwatch. You may also report side effects to Genentech at (888) 835‑2555.
Please see full COTELLIC Prescribing Information for additional Important Safety Information.

